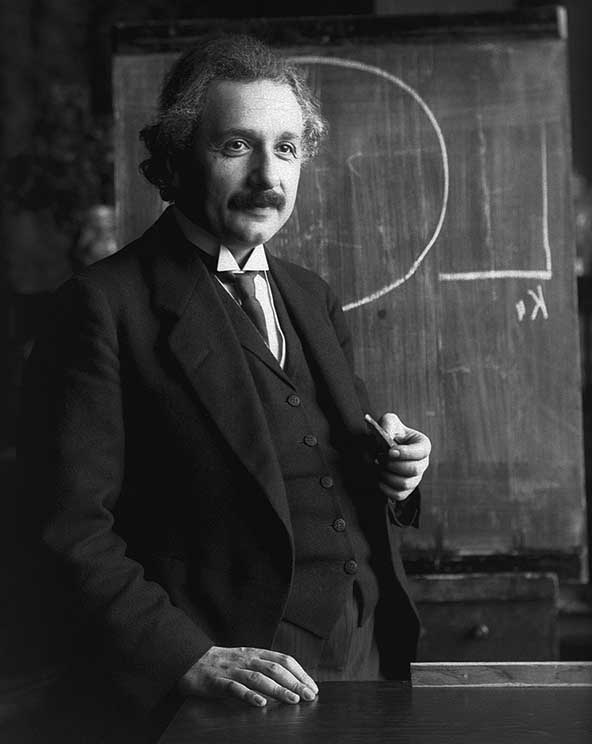Niels Bohr ur.1885- died 1962

Quantum mechanics is the foundation of twentieth-century physics. It made it possible to understand the phenomena occurring in the microworld and allowed for many technical achievements, such as the construction of the transistor, microprocessor and the mastery of nuclear energy. Thanks to quantum mechanics, we better understand the structure of chemical bonds and many biological phenomena, and thus we have new possibilities to manipulate nature. Even in cosmology, quantum ideas are important today. Quantum mechanics has not only caused enormous changes in our daily lives, but it also forced many changes in philosophical views. The Dane Niels Bohr was the most eminent physicist among these, who took part in the development of quantum theory.
Niels Bohr played a decisive role in the transformation, to which physics underwent in the 20th century. About 1913 R. developed a model of the atom, which has gained great recognition, and in the mid-1920s he participated in the birth of a new quantum theory – mathematical interpretation of intra-atomic reality – which has remained the dominant theory in physics to this day. quantum physics, and especially the so-called Copenhagen interpretation of the new theory, was a huge success, Bohr's influence was decisive for its acceptance. All the great achievements of chemistry and electronics, and the development of nuclear power, are derived from quantum theory. Its consequence is also the present rapprochement of physics, cosmology and biology. The great importance of quantum theory is also due to its philosophical implications. Niels Bohr put an end to his earnest quest to discover "ultimate" reality. According to Bohr, “The view is a mistake, that the job of physics is to detect, what is nature. Physics is about it, what can we say about nature ".
Niels Bohr was born in Copenhagen 7 October 1885 R. He was the son of Christian Bohr, professor of physiology, and Ellena née Adler. The Bohrs were very close. They formed a cultural family, with a high intellectual level, thus, Niels grew up in an environment conducive to the development of his genius. The mother was cordial and intelligent. Father was careful, as Bohr himself later recalled: "That you should expect something from me". The Bohrs were not very pious and Niels became an atheist. He was careful, that religious beliefs are wrong and harmful. From 1891 R. attended Gammelholms Latin og Realskole, where he was remembered as a good student, a boy tall for his age, prone to fights, but also a bit shy. As he recalls, he was passionate about science "thanks to his father's influence". W 1903 R. entered the University of Copenhagen, where he mainly dealt with physics. He obtained a master's degree in 1909 R., and the doctor in 1911 R. In the same year, his father died, and Niels married Margeth Norlund.
Revolution in views on the construction of the atom in 1911 R. it was already a fact. Bohr's PhD thesis was about the theory of electrons, discovered about ten years earlier by J.. J. Thomson. It was known, that electrons are a common component of matter. Thomson also suggested, that the number of electrons in an atom corresponds to its mass and determines the diversity of the atoms of the permanent elements. Ernest Rutherford has demonstrated, that the atom is small, heavy testicle, which, of course, was of fundamental importance. The discovery made it, that physicists deviated from the theory, according to which the atom was something like a raisin pudding – consisting of a nucleus dotted with electrons like raisins in a cake – in favor of the Rutherford model, in which electrons revolve around a tiny nucleus.
W 1913 R. Bohr, working in England with Rutherford, published three works on the structure of the atom, which definitely influenced the further development of physics. The Rutherford model did solve some important problems, however, there was still no answer to the basic question: why electrons – certainly attracted by the nucleus – they are not finally absorbed by them. In short, the Rutherford model did not explain the stability of the atom, one of its basic features.
Bohr understood, that classical Newtonian mechanics cannot explain the behavior of matter on the atomic scale, therefore he became interested in the quantum theory of "blackbody radiation", formulated at the turn of the century by Max Planck, which Einstein used to explain the behavior of "particles" of light. W 1912 R., after a relatively short period of hard work, Bohr explained, why the hydrogen atom emits light rays, and developed a theory that corresponded very well to the experimental facts. Bohr founded, that the electron only radiates light then, when it changes its orbit, that is, the emission of a quantum of light accompanies the "jump" of an electron from one orbit to another. Einstein, having learned about the results of Bohr's work, he said confidently, with the laconic nature of it: "This is a tremendous achievement".
Building a model of the atom, called the Rutherford-Bohr model, it was a great step forward and was soon used to explain the atomic structure of all the other elements. One of Bohr's achievements in 1913 R. was the identification of the X-ray spectrum with the corresponding quantum electron hopping. Spectroscopy made it possible for nineteenth-century scientists to discover and study many elements. X-rays, having a much shorter wavelength than visible light, provide important information on phenomena occurring at the atomic scale. Father Gustav Kirchhoff i Max von Laue.
Over the next year, British physicist Harry Moseley developed the, under the direction of Bohr, on the basis of X-ray spectrum studies of individual elements, new, the final order of the elements on the periodic table and assigned each element the correct atomic number. Over the next few years, Bohr made many more detailed discoveries. As Abraham Pais wrote: "Judging now… it was all the more fabulous and amazing, that it came from an analogy – atomic orbits similar to the orbits of the planets orbiting the sun, mole (spin) similar to the rotation of the planets around their axis – although in reality these analogies are false ". W 1922 R. Bohr was awarded the Nobel Prize.
Turned out, that, in fact, the Bohr model of the atom had some serious shortcomings. The so-called first quantum revolution did not solve all the problems related to the behavior of more complex atoms. Even though in patches 1913-1925 this theory has been adjusted in various ways, serious doubts grew at the same time, which eventually led to the "second quantum revolution". In the 1920s. Bohr played a decisive role in solving the crisis in physics, caused by the disadvantages of the atomic model, which he made himself. Having returned in 1916 R. to the University of Copenhagen, Bohr became professor of theoretical physics and five years later participated in the opening of the Institute of Theoretical Physics. Thanks to Bohr, Copenhagen has become a center of attraction for physicists. The so-called second quantum revolution contributed to the emergence of a new one, purely mathematical model of the atom, which in fact confirmed the limited human ability to perceive intra-atomic phenomena. The second revolution consisted of Schrodinger wave mechanics and Heisenberg's matrix mechanics and his famous uncertainty principle, which defines the limits of cognition of physical systems.
In the late 1920s, Bohr developed two principles, that would help bring the quantum revolution to a happy end. In a famous lecture by St. 1927 R. pt. "The Philosophical Foundations of Quantum Theory" Bohr discussed the concept of complementarity for the first time. Its essence is, that however atomic systems can be defined in a mutually contradictory manner – as waves and as particles – both of these features are necessary for an exhaustive description of phenomena. Bohr intrigued by the philosophical implications of this concept, eventually he came to a conclusion, that the principle of complementarity relates to the problem of free will and the basic processes of life. Perhaps a more important corollary to this principle was this, that quantum theory was used to exhaustively describe nature, which later discoveries did not change. There is no "deeper" reality behind quantum measurements, and although this concept was later often attacked from all sorts of sides, it remained the foundation of the "Copenhagen spirit" – despite various mental experiments, considerations on the "mind of God" and the theory of multiple universes. The doctrine of complementarity was never fully recognized by Albert Einstein, ani Max Planck, nor many other physicists, but nevertheless it remains the basis of physics to this day.
In the 1930s, Bohr became involved in nuclear physics. W 1934 R. proposed a droplet model of the atomic nucleus, which turned out to be very helpful in understanding the phenomenon of nuclear fission. W 1936 R. formulated the theory of the atomic nucleus, which served as the basis for further nuclear research in the next decade. According to Bohr's theory, neutrons and protons tightly packed in an atomic nucleus are bound by a strong interaction, which counterbalances the mutual repulsion of protons, having the same electric charge. Admittedly it was obvious, that the imbalance of the nucleus would be accompanied by the release of energy, but it was far from physicists to understand all the consequences of this process.
After the outbreak of World War II, Bohr initially remained in Denmark, which the Germans took in 1940 R. Due to his position, he managed to save many of his colleagues from persecution, however, he refused to cooperate with the Germans. W 1943 R. Rumors spread about the imminent imprisonment of Bohr. The scholar and his family fled to Sweden then, and then to England, then he found himself in the United States. He soon joined the team working on the Manhattan Project. For security reasons, he was given the nickname Uncle Nick (Uncle Nick). Bohr's participation in the project was more symbolic than real. Bohr was against the dropping of the atomic bomb. During the war, he met Roosevelt and Churchill, who rejected his offer, that information on nuclear research be provided to the Soviet Union in order to prevent a nuclear arms race.
After returning to Denmark, Bohr worked actively until the end of his life. He retired from the university in 1955 R. As a scientist, he was involved in the fight against nuclear weapons; he wrote, inter alia, in 1950 R. the famous "open letter" to the United Nations. He has received many awards, including the "Atom for Peace" award by 1957 R. He actively supported international cooperation in the field of physics research and contributed to the creation of the European Nuclear Research Center (CERN) in Geneva. 17 November 1962 R. gave the last interview on the history of quantum theory. Next day, during her usual after-dinner nap, he died of a heart attack. He was buried in the family grave in Copenhagen.
Bohr was widely recognized, some of the statements make it easy to understand, how great was its importance. This is how Victor Weisskopf described the atmosphere in Bohr's "Copenhagen school": “We saw him, the greatest of our colleagues, how he worked, he was talking, he was alive. He was like most young people – optimistic, cheerful, enthusiastic, attacking the deepest mysteries of nature without being bound by conventional ties, with a feeling of joy, which is difficult to describe ". Whatever the style, as an expression of adoration, Weisskopf's remarks are accurate. Abraham Pais agreed with them, who wrote: “Bohr played a major role in explaining the changes in the philosophical tenets of physics, necessary to understand quantum phenomena ". Richard Rhodes put it more simply: "Bohr's contribution to the physics of the twentieth century. second only to the achievements of Einstein ".