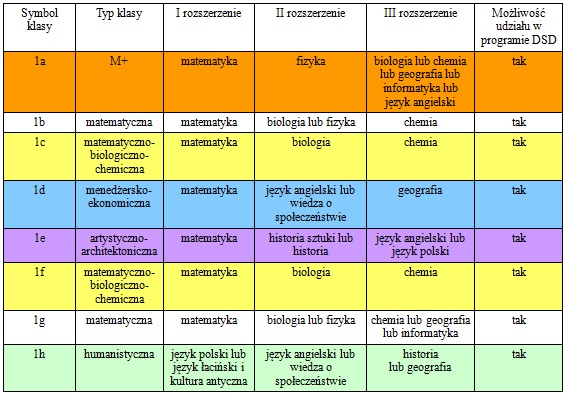
What are the properties of carbon
Properties of coal
The question is certainly not about coal, which we burn in stoves, but carbon as an element. You could say, that coal has a very ambivalent nature, because it can be found both in inorganic chemistry (acids, sole itd.) as well as in organic chemistry (the main organic element, np. in alkanes, sugars, lipids, proteins etc.). Like every element, carbon also has physical and chemical properties. Of course, we should start with the physical properties of coal.
Carbon is a solid. It does not dissolve in water! Moreover, coal has neither any flavor nor smell. Everyone knows too, that the charcoal is black. As for the chemical properties, you need to write about it, that carbon forms such compounds, where its valence is either II as in carbon monoxide CO or IV as in carbon dioxide CO2. Carbon reacts with water to form a very weak carbonic acid. Coal is combusted to form carbon dioxide or very dangerous carbon monoxide (II) that is, the so-called chad, which threatens human health and life.
In nature, carbon comes in several varieties. They are the so-called. allotropy is, that is a diamond, graphite and fullerene. It is mined as hard and brown coal. Carbon black is the amorphous form of carbon. It is an element common all over the globe.